Describe a Solution Using the Words Solute and Solvent
Conductive solutions always result from the presence of a solute that is ionic meaning it dissociates in water into charged particles called ions. Also he was the first to scientifically describe the process 39.

Solute And Solvent Ck 12 Foundation
As such it compares estimates of pre- and post-test probabilityTo make the context clear by the.

. 6 δ s s s o l u t e s s o l u t i o n where s solute and s solution are the average interatomic spacings for the solute atom and corresponding solvent solution. Thats a lot of sol words. Calculate the enthalpy of solution of Na2SO4 in water at this concentration.
These can be read as parts eg 1 part in 100 parts. A solution changes depending on the concentration and kind of solute in the solution. In ratio strength the first number is a 1 and it is followed by a colon and then another number eg 1100.
The calorimeter constant is 330 J K-1. Three changes of buffer solution within 24 h using a 5 mL Float-A-Lyzer dialysis tube with a 3500 Da cutoff. As a control for the temperature change we placed the same amount of solute in the same amount of solvent and let the solution stand for five minutes without heating it Structure and style.
Freezing point depression is not unique to water and salt. How much solute is dissolved in a certain amount of solvent. Solubility is the amount of a solute needed to form a saturated solution at a specific temperature and specific solvent amount.
As the protein-buffer solutions were diluted during dialysis. In other words in two extractions using the same 100 cc ether we can separate 23 29 or 889 of the original amount of the compound. To define a solution precisely we need to state its concentration.
The minor component in a solution dissolved in the solvent. It is usually accompanied by a favorable increase in the entropy of the solvent. The amount of solute that can be dissolved by the solvent is defined as solubility.
They also have names for the different types of homogenous mixtures. To make a solution you dissolve a solute in a solvent. You will also compare the results from your experiments with the results predicted by.
Solution is the general term used to describe homogenous mixtures with small particles. Words such as dilute or concentrated are used to describe solutions that have a little or a lot of dissolved solute respectively but these are relative terms with meanings that depend on various factors. But if the solution is composed of two or more components the boiling point of the solution is different from the pure solvent and is called the bubble point.
The Results and Discussion section describes our experimental observations. Ratio strength can be used to describe the concentration of a dilute solution. All cells are surrounded by a lipid bi-layer cell membrane which permits the flow of.
A liquid mixture in. The theoretical background of modern liquid chromatography was first devised by the two British chemists who were the 1952 Nobel Prize winners AJP. At the bubble point of a liquid the ratio between the mole fraction of solute in the vapor phase and the mole fraction of solute in the liquid phase is equal to the distribution.
Martin 19102002 and RLM. Accordingly the interatomic spacing difference δ s between the solute and its solvent solution for each pseudo-binary solution is expressed as. Organization is especially important in the Methods section of a lab report because readers must understand your experimental procedure completely.
Colloids Science has special names for everything. The solvent does. Accuracy is also used as a statistical measure of how well a binary classification test correctly identifies or excludes a condition.
The units are always grams or milliliters depending upon whether you are. Depending upon the solute the resulting solution will either conduct electricity or be non-conductive. Osmosis is the process in which water flows from a volume with a low solute concentration osmolarity to an adjacent region with a higher solute concentration until equilibrium between the two areas is reached.
You assign the units. P422 A sample of Na2SO4s is dissolved in 225g of water at 298 K such that the solution is 0200 molar in Na2SO4. A temperature rise of 0101ºC is observed.
Gradient elution involves using a weak eluting solvent at the start of. Compare your result with that calculated using the data in Table 41. After this Introduction we briefly describe the experimental and theoretical methods used.
That is the accuracy is the proportion of correct predictions both true positives and true negatives among the total number of cases examined. It happens with all solutions.
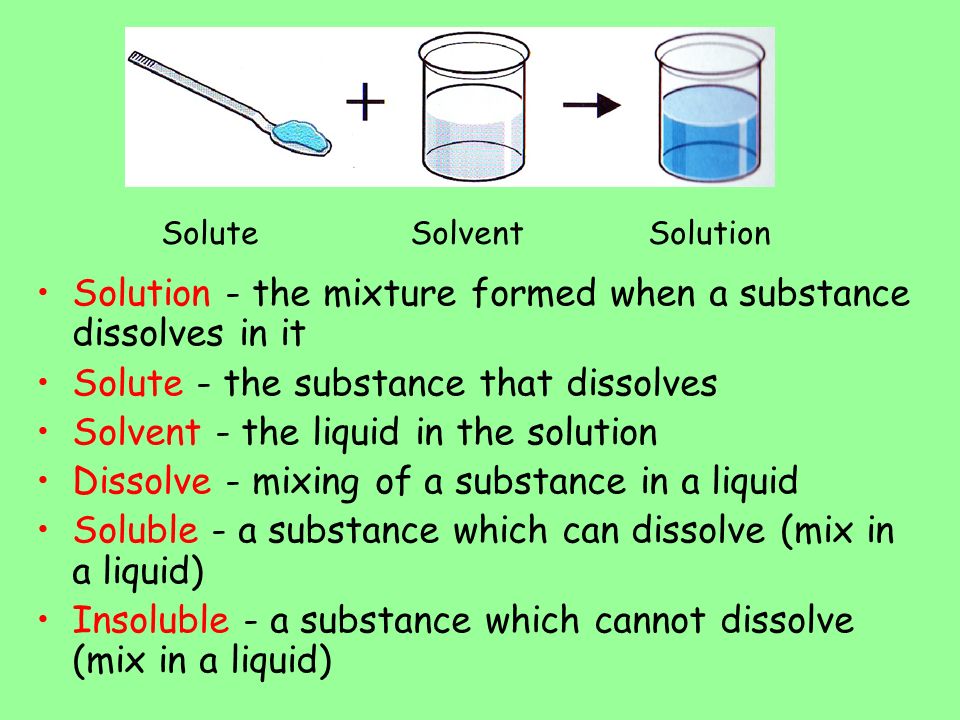
Solutions Solvents Solutes Ppt Video Online Download

Year 7 Science Lesson Solute Solvent Solution Edplace Youtube

Solute Vs Solvent Definition Difference Between Solute And Solvent With Examples
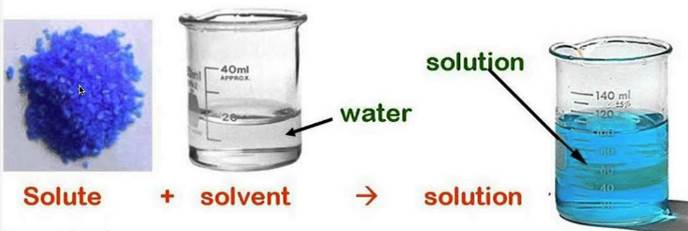
Comments
Post a Comment